Buechel - Pappas ™
Primary Knee System
TiN coated with Ultracoat®
As the primary refinement, for all the metal components of B-P™ knee is coated with advanced coating method to increase the wear and scratch resistance.
- Titanium Femur and Tibia
- Lighter, Stronger and Long lasting
- The coating method is TiN ceramic Ultracoat®
- High Flex Design
- Deep Dish Design for Low Contact Stress
- Mobile Bearing platform
- Stop Pin in Tibia to prevent Spin out
We Balance Gaps!
Overview
- 4th Generation New Jersey Knee
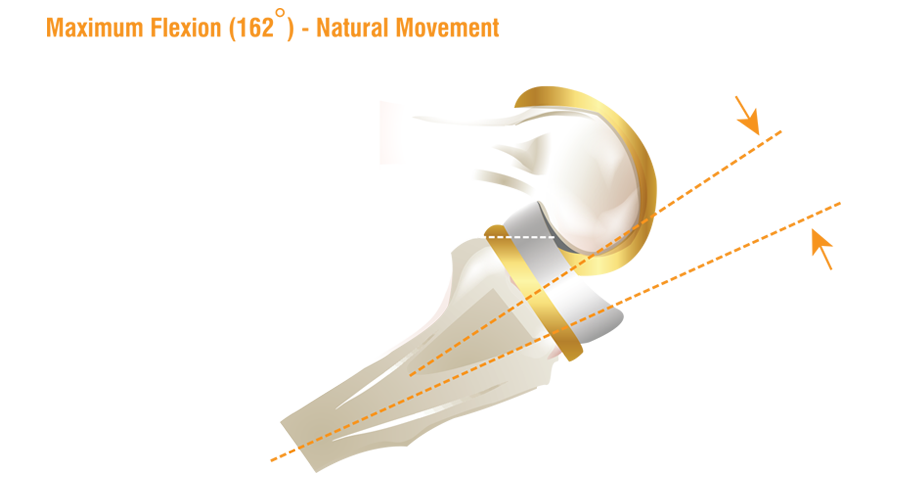
- High Flex Design
- Designed for ROM upto 162° Flexion
- Ultra congruent Deep Dish Design for Low Contact Stress
- Mobile Bearing platform
- Stop Pin in Tibia to prevent Spin out
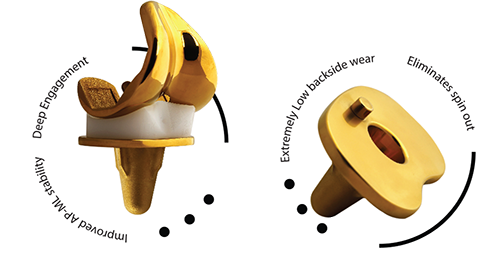
About Buechel Pappas TiN Coated Knee
Based on experience with the LCS, Dr.Buechel and Pappas have refined the design and have came out with B-P™ Total Knee System
As the primary refinement, Titanium components (Femur and Tibia) of the B-P™ knee is coated with an advanced coating method to increase the wear and scratch resistance.
The coating method is patented TiN ceramic UltraCoat® from Ionbond IHI Group, UK
Design Rationale
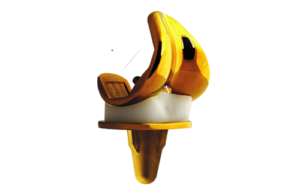
Buechel & Pappas has developed a set of "Narrow" femoral components, which are 7.6% narrower than the standard components for use.
The B-P™ TiN Coated Femoral Component avoids gender specific characteristics, such as a lower femoral flange and lack of normal axial rotation, of some of the competitive designs. A lower femoral flange increases the risk of patellar subluxation, a problem much more common in females than in males. The inability to provide normal axial rotation generates unnecessary forces on the knee implants and bone-/implant interfaces increasing the risk of wear and loosening.
Ultracoat® Technology
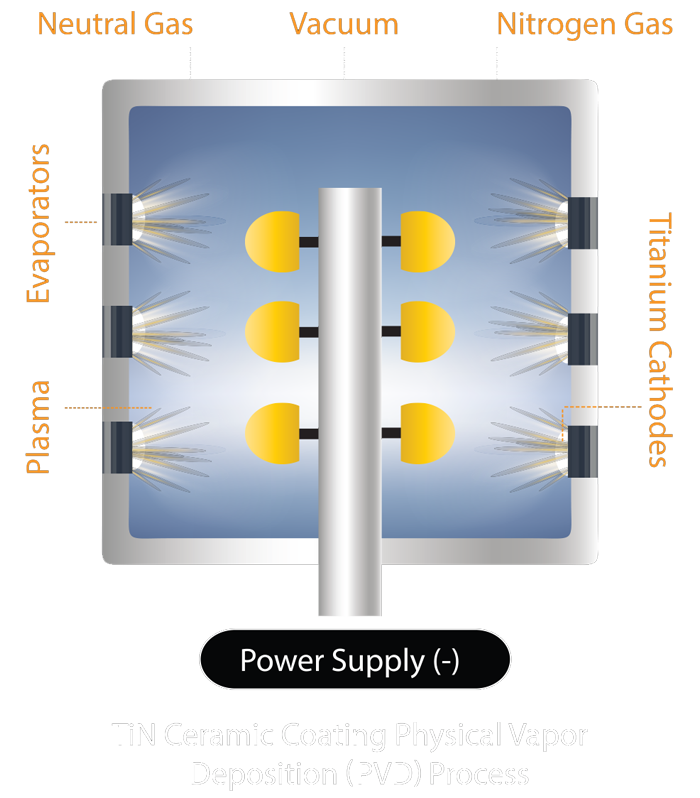
ULTRACOAT® is a result 15 years of research, development and clinical evaluation offers a thoroughly tested coating. Not all titanium surface treatments are the same. It is imperative to utilize a coating that has been thoroughly tested.
Distinctive Attributes
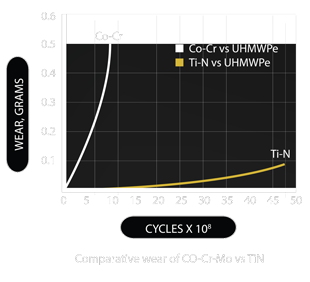
- Ultracoat® the TiN coat that has been thoroughly tested
- Ultracoat® on the articulating surfaces reduces the wear and tear
- The PVD TiN ceramic coating has an average thickness of 4 microns and it is harder and smoother than Co-Cr
- It is extremely hard ( 3000 Vickers ); biologically inert with low friction properties1-7
- Ceramic coating provides a significantly better environment for biological fixation than uncoated metal surface
- The durability of the TiN coating has been demonstrated by a 48 million cycle test of femoral resurfacing components8
Clinically Proven
Clinical Affirmation (1991 - 2004 follow up)
Comparison of TiN Cermaic Coated knees with Co-Cr implants
| KNEE TYPE | Co-Cr[9] | B-P TiN COATED[10] |
|---|---|---|
| Number in study | 120 | 76 |
| Time in situ | 5-17.3 years | 3-14.4 years |
| Knee score (NJOH) | 87.3% | 92% |
| ROM | 0-107 deg | 0-116 deg |
| Patient satisfaction | 94% | 97% |
| Polyethylene wear | 3 (1.8%) | 0 |
| Osteolysis | 3 (1.8%) | 0 |
| Recurrent synovitis | 1 (0.6%) | 0 |
| Dislocation/ subluxation | 2 (1.2%) | 0 |
The Ceramic Coated B-P Knee is superior to Co-Cr across parameters especially with respect to wear related complications
Extra Resources
| Buechel Pappas Brochure TiN Knee Product Manual | |
| Buechal Pappas Surgical Steps Illustrated | |
| BP Knee Surgical Steps |

References
- Bolster, RN et al; Tribiological behaviour of TiN Films deposited by high energy ion-beam-assisted deposition; Surface and Coating Technology Volume 36, Issues 3-4, 15 December 1988, Pages 781-790
- McKellop, H el al, "Friction and wear properties of ploymer, metal and ceramic prosthetic joint materials evaluated on a multichannel screening devices"; Journal of biomedical materials research, Vol.15, 1981.
- Johansen OA et al; Reactive arc vapour ion deposition of TiN, ZrN and HfN; Thin Solid Flims Volume 153, Issues 1-3, 26 October 1987, Pages 75-82
- Holleck h; Material selection for hard coatings, Journal of Vacuum Science & Technology A 4, 2661 ( 1986 )
- Coll BF et al; Surface modification of medical implants and surgical devices using TiN layers; Surface and coating Technology Volume 36, Issues 3-4, 15 December 1988, Pages 867-878
- Black J; Biological Performance of Materials, Fundamentals of Biocompatibility, Fourth Edition; https://oi.org/10.1201/9781420057843.
- Hayashi K et al; Evaluation of metal implants coated with several types of ceramics as biomaterials; Journal of Biomedical Materials Research banner; Volume 23, issue 11
- Pappas MJ, Makris G, Buechel FF; Titanium nitride ceramic film against polyethylene - A 48 million cycle wear test; Clinaical Orthopaedics and Related Research [01 Aug 1995 (317):64-70]
- Buechel FF et al; CORR 2002
- Buchel FF et al;"The Buechel-Pappas total knee: design improvement from the LCS" presented at 32nd OST meeting, Heron Island, Australia, July 3-9 2006