TTK Healthcare Ltd., has transcended the national boundary and entered into various technical agreements and international collaborations in order to provide global standard implants in Joint Replacement arena.
Buechel Pappas Trust

Frederick F. Buechel and Michael J. Pappas are the founders of the BP™ trust and have significantly contributed to the growth and development of Joint Replacement Technologies. Buechel is a prominent orthopedic surgeon and Pappas is a renowned expert in advance design.
The TTK Ortho Division has a technical collaboration with Dr.M.J.Pappasand Dr.F.F. Buechel's M/s. BP Trust for the manufacturing of Total Knee, Hip, Shoulder and Ankle joints. These designs are US FDA approved and are a result of over 25 years of development, clinical investigation and use.
About Frederick F. Buechel, M.D., F.A.A.O.S., F.A.C.S.
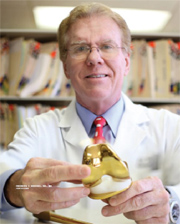
Dr. Buechel is a high-profile orthopaedic surgeon, researcher and developer in the field of total joint replacement since 1974. He has gained international recognition for his developments and has collaboratively presented and published numerous scientific articles in the field of total joint reconstructive surgery. This includes more than 150 research articles and more than 100 United States and international patents in the area of joint replacement implant and instrument design.
Dr. Buechel is recognized for his pioneering work in the area of mobile-bearings, which includes the development of the world's leading mobile-bearing knee, the New Jersey LCS® Total Knee System. He is also very proud of the outstanding clinical results of the Buechel-Pappas Total Knee, which is the latest improvement over the LCS Knee.
About Michael J. Pappas, Ph.D, P.E.

Dr. Pappas was an internationally recognized expert in advanced design. He received his Ph.D. in 1970 from Rutgers University in Mechanical Engineering specializing in Computer-Aided Structural Design. His research and publications in this field attracted international attention.
He has been a professor of Mechanical Engineering at New Jersey Institute of Technology for more than 25 years. Dr. Pappas was a Principal of an industrial design firm for more than 20 years during which time he helped design a number of industrial and consumer products.
EDGE International , USA

Edge International, with facilities in Dayton, Ohio, USA, is committed to providing products that meet all requirements as defined by the customer, with on-time delivery of those products. Edge International is certified to ISO 13485:2016 for its Quality Management System. There are no geographical restrictions on Edge International's operating base. In addition to the United States, the company transacts business internationally with customers in Asia, Europe, and North and South America.
For more Information kindly visit www.edgeintl.com
Endolab Mechanical Engineering GmbH, Germany

EndoLab is one of the biggest and most established testing laboratories worldwide with more than 25 years of experience. As a key partner of the industry, we are connected to authorities and private companies alike. By contributing to new testing standards, we shape the industry’s future and ensure we are always in tune with the latest developments.
For more Information kindly visit : www.endolab.org
Lincotek S.p.A, Italy
Plasma Spray Hydroxyapatite (HA) coatings include Osprovit® and Titanium Plasma Spray (TPS) coatings include Ti-Growth® on Hip Implants are done from Lincotek Trento S.p.A , Italy.
About Lincotek Trento S.p.A :
Lincotek, headquartered in Rubbiano, Italy, is a global contract manufacturer for markets including Industrial GasTurbine, Aviation and Medical Device applications. The Group is one of the most respected producers in the Additive Manufacturing market worldwide due to its talented team and production capabilities. Lincotek Medical is the medical division of Lincotek, a family-owned Group that has served global markets for more than 45 years. The group has more than 1,100 employees located in 16 production facilities across Europe, North America and Asia.
We take meeting medical standards very seriously and ensure that all of our facilities meet the highest appropriate requirements. All of our production sites for the medical field meet the International Organization for Standardization (ISO) certification ‘Medical Devices – Quality Management Systems – Requirements for Regulatory purposes’ (ISO 13485). We meet CE marking specifications for medical devices (classes IIa, IIb and class III bio-resorbable).
For more Information kindly visit www.lincotekmedical.com
Orthoplastics

Orthoplastics is fully dedicated to the medical sector, producing the highest grade of orthopaedic UHMWPE products on the market.
A combination of rigorous quality control systems ensures that Orthoplastics continually exceed the Documented Quality System requirements of International Standards, including surpassing the specifications of 21 CFR 820. Orthoplastics have been accredited to ISO 9001, ISO 13485 and ISO 14001, which are all regulated under Validation Control (IQ, OQ/PQ) and FMEA studies.
Today, Orthoplastics is recognised globally as the leading supplier of UHMWPE, supplying many of the world’s orthopaedic device manufacturers. Its premium grade UHMWPE is expertly manufactured onsite in the UK and is the material of choice in a wide range of orthopaedic implants and devices for applications in hips, knees, shoulders, elbows, ankles and fingers.
For more Information kindly visit www.orthoplastics.com